Tracing the metal in wood
14 March 2022Dr Simon Curling, a research scientist at the BioComposites Centre, Bangor, looks at the methods available to check trace metals in timber
As the use of recycled wood in panels increases there is an obvious need to determine just what is in the raw material. Large contaminants such as nails or stones are generally easy to spot, but what about treatments or paints?
As older or reclaimed wood is reused, the need to recognise toxic and heavy metals in the raw material is vital. Although, of course, it must be remembered that fresh wood can also contain some heavy metals if the trees are growing on soils that are naturally or artificially high in those elements.
Clearly, it’s important for products to be low in these heavy metals but how can they be detected? With experience, treated or painted wood can be identified in a batch, but this doesn’t help answer the question of what levels of trace metals are there.
One common technique is the grand sounding method Inductively Coupled Plasma – Optical (or Atomic) Emission Spectrometry, which is thankfully shortened to ICP-O(A)ES. This technique is used in a number of fields, ranging from soil analysis to quality control in food and cosmetics. In the timber products industry, it is an ideal method to determine natural occurring trace metals and those present due to wood preservatives, paints, or coatings.
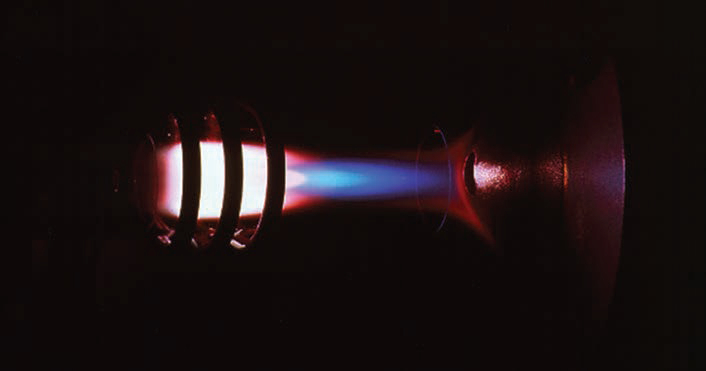
This is a lab-based method that uses plasma, at temperatures of 6,000 to 10,000 Kelvin (pictured below), to excite the metal ions and atoms within a solution to emit electromagnetic radiation. The wavelength of the radiation is specific to a certain element; for example, for iron (Fe) one of the emission wavelengths is 238.204nm, whilst for lead (Pb) it’s 220.253nm and for copper (Cu) 324.754nm. The intensity detected is dependent on the concentration of the elements, so by measuring both wavelength and intensity and comparing these to a calibration sample the concentration can be determined. Modern machines will analyse the elements in a solution simultaneously, resulting in measurement times of one to two minutes per sample.
The method isn’t suitable for determining concentrations of all of the elements of the periodic table but will cover the elements of interest to the industry.
The method will detect low levels of metals and although detection levels are not the same for all elements, concentrations in the parts per billion are detectable given the careful preparation, with some brightly emitting elements. For example, calcium, magnesium, or barium, are detectable at levels of tens of parts per trillion. However, in terms of quality control, detection in parts per billion is more than adequate.
The method is clearly destructive, and it is simply not feasible to test every scrap of wood that is used. However, with good operating procedures a good level of quality control can be ensured.
For example, a systematic sampling regime to sample loads as they arrive should give a good representation of the quality of the batch. If a batch looks to be of concern, with painted or treated material apparent, a more robust sample regime may be required.
These samples then need to be prepared, with cutting and grinding the material into dry powder the first step. Then, small amounts of the powder need to be chemically digested with acids to break the material down and get it into solution.
There are several methods that can be used, depending on the material. However, an effective method for wood is to use hot nitric acid and hydrogen peroxide.
To speed the digestion up and to ensure reproducibility of the results, microwave digestion systems can be used. These are commonly used to prepare a wide range of environmental substrates for testing.
So, whilst ICP-OES needs well devised sampling strategies, good sample preparation and a trained user for full effectiveness, it is clearly a powerful tool in determination of trace metals in materials.